Abstract

Introduction:
Myelofibrosis (MF) is a myeloproliferative neoplasm (MPN) characterized by splenomegaly, constitutional symptoms, bone marrow fibrosis, and a propensity toward transformation to acute myeloid leukemia. Type I JAK inhibitors (JAKi) are the only approved therapy and can provide symptom control and reduction of splenomegaly in eligible patients. However, JAKi do not significantly impact disease progression, and many patients are not eligible due to coexisting cytopenias. Patients with MF who have relapsed and/or are refractory (R/R) to JAKi have poor survival. Therefore, novel agents, moving beyond JAKi-based treatments, are urgently needed. CD123, also known as the interleukin-3 receptor alpha chain, is expressed on maturing myeloid cells and progenitors in MF, making it an attractive therapeutic target. Tagraxofusp (TAG, SL-401) is a CD123-targeted therapy which is FDA and EMA approved for the treatment of blastic plasmacytoid dendritic cell neoplasm. A phase 1/2 study of TAG monotherapy in patients with MF who were R/R to approved JAKi (NCT02268253) was initiated. Herein, we present updated safety and efficacy data.
Methods:
A 2-stage multicenter, phase 1/2 study enrolled patients with R/R MF. Patients in Stage 1 (dose escalation) received 7, 9, or 12 mcg/kg/day TAG intravenously on days 1-3, every 21 days (cycles 1-4), every 28 days (cycles 5-7), and every 42 days (for cycles 8 and beyond); 12 mcg/kg/day was given every 21 days (cycles 1-4), and every 28 days (cycles 5+) in Stage 2. Enrollment in Stage 2 (expansion) was restricted to patients who met the DIPSS-plus intermediate-2 or high-risk disease criteria, those who were R/R or intolerant to JAKi therapy, and those who were not eligible for immediate stem cell transplantation. Responses were assessed on the basis of the International Working Group MPN Research and Treatment/European LeukemiaNet (IWG-MRT/ELN) criteria (Tefferi et al. Blood 2013;122:1395-1398).
Results:
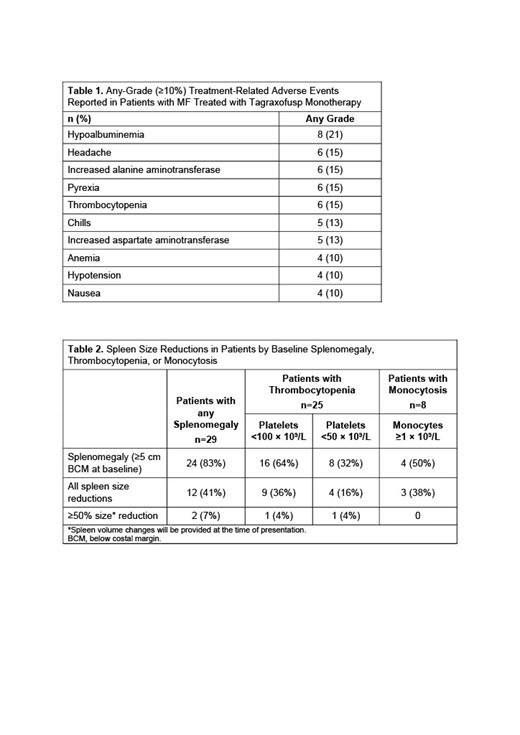
As of July 1, 2021, 39 patients with MF were treated. Median age was 70 years (range 54-87) and 46% were female. The baseline characteristics comprised thrombocytopenia (<100 × 10 9/L; 64%), including 36% with platelets <50 × 10 9/L; monocytosis (≥1.0 × 10 9/L; 21%), and splenomegaly (74%). Genomic assessments at study entry demonstrated molecular mutations in JAK2, CALR, MPL, and ASXL1 in 62%, 8%, 5%, and 23%, respectively; cytogenetic abnormalities were observed in 10 patients (4 patients: 20q-, 3 patients: 7/7q-, 2 patients: -5/5q-, 2 patients: 12p-, and 1 patient: 17q). Treatment-related adverse events are depicted in Table 1. The most common treatment-related adverse events (incidence ≥10%) included hypoalbuminemia (21%), headache, pyrexia, thrombocytopenia, and elevated alanine aminotransferase (15% each). Capillary leak syndrome (CLS) was reported in 3 patients, including 1 patient with grade 4; there was no grade 5 CLS. Stable disease was achieved in 21 patients (60%). In total, 12/24 patients (50%) with baseline splenomegaly achieved a spleen response (Table 2), 3/4 (75%) with concomitant monocytosis, and 13/24 (54%) with concomitant thrombocytopenia. Spleen responses were confirmed objectively by imaging, with the spleen volume reduction (SVR) thresholds for response set at 35%. Updated information on SVR will be reported at the time of presentation. Total Symptom Score (TSS) was reduced in 20/39 patients (51%), and 10/39 (26%) had reductions from baseline in both TSS and spleen size. Median overall survival was 29.2 months (95% CI 12.0-49.5; range 1-51 months).
Conclusions:
TAG monotherapy demonstrated clinical efficacy and a predictable and manageable safety profile in patients with MF characterized as high-risk, including those who are refractory to JAKi and those with associated monocytosis, thrombocytopenia, advanced disease, and high-risk genetic features. This phase 1/2 study confirms the safety and clinical efficacy of TAG administered as a monotherapy in a cohort of poor-prognosis patients with MF. Given the genomic and epigenomic complexity of MF, with the clonal heterogeneity and additional subclones, TAG is planned to be tested as part of a combination strategy in high-risk patients with MF who are either R/R, intolerant to, or who have had a prior suboptimal response to JAKi.
Yacoub: Agios: Membership on an entity's Board of Directors or advisory committees; Acceleron Pharma: Membership on an entity's Board of Directors or advisory committees; CTI Biopharma: Membership on an entity's Board of Directors or advisory committees; Novartis: Membership on an entity's Board of Directors or advisory committees; Incyte: Speakers Bureau. Patnaik: Kura Oncology: Research Funding; Stemline Therapeutics: Membership on an entity's Board of Directors or advisory committees; Stemline Therapeutics: Membership on an entity's Board of Directors or advisory committees. Ali: Incyte: Membership on an entity's Board of Directors or advisory committees, Speakers Bureau; BMS: Speakers Bureau; CTI BioPharma: Membership on an entity's Board of Directors or advisory committees. Wang: Pfizer: Consultancy, Honoraria, Other: Advisory Board, Speakers Bureau; Takeda: Consultancy, Honoraria, Other: Advisory board; AbbVie: Consultancy, Membership on an entity's Board of Directors or advisory committees; GlaxoSmithKline: Consultancy, Honoraria, Other: Advisory Board; Stemline Therapeutics: Consultancy, Honoraria, Other: Advisory board, Speakers Bureau; Genentech: Membership on an entity's Board of Directors or advisory committees; Astellas: Consultancy, Membership on an entity's Board of Directors or advisory committees; DAVA Oncology: Consultancy, Speakers Bureau; BMS/Celgene: Membership on an entity's Board of Directors or advisory committees; Jazz Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Kite Pharmaceuticals: Consultancy, Honoraria, Other: Advisory Board; Kura Oncology: Consultancy, Honoraria, Other: Advisory board, steering committee, Speakers Bureau; Novartis: Consultancy, Honoraria, Other: Advisory Board; Mana Therapeutics: Consultancy, Honoraria; Rafael Pharmaceuticals: Other: Data safety monitoring committee; Gilead: Consultancy, Honoraria, Other: Advisory board; Daiichi Sankyo: Consultancy, Honoraria, Other: Advisory board; PTC Therapeutics: Consultancy, Honoraria, Other: Advisory board; Genentech: Consultancy; MacroGenics: Consultancy. Gupta: Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; AbbVie: Consultancy, Honoraria; BMS-Celgene: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Incyte: Honoraria, Research Funding; Roche: Consultancy; Constellation Pharma: Consultancy, Honoraria; Sierra Oncology: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Pfizer: Consultancy. Lee: BMS: Consultancy, Membership on an entity's Board of Directors or advisory committees; Innate: Consultancy, Membership on an entity's Board of Directors or advisory committees; Pin Therapeutics: Consultancy, Membership on an entity's Board of Directors or advisory committees; AstraZeneca: Consultancy, Membership on an entity's Board of Directors or advisory committees. Schiller: FujiFilm: Research Funding; Deciphera: Research Funding; Daiichi-Sankyo: Research Funding; Constellation Pharmaceuticals: Research Funding; Celator: Research Funding; BMS/Celgene: Consultancy, Current equity holder in publicly-traded company, Research Funding, Speakers Bureau; Amgen: Consultancy, Current equity holder in publicly-traded company, Honoraria, Research Funding, Speakers Bureau; Karyopharm: Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Actinium Pharmaceuticals, Inc: Research Funding; Samus: Research Funding; Stemline Therapeutics, Inc.: Honoraria, Research Funding, Speakers Bureau; Agios: Consultancy, Research Funding, Speakers Bureau; Geron: Research Funding; Bluebird Bio: Research Funding; Kite/Gilead: Honoraria, Research Funding, Speakers Bureau; Mateon: Research Funding; Onconova: Research Funding; Jazz: Consultancy, Honoraria, Research Funding, Speakers Bureau; PrECOG: Research Funding; Regimmune: Research Funding; Pfizer: Current equity holder in publicly-traded company, Research Funding; Genentech-Roche: Research Funding; Gamida Cell Ltd.: Research Funding; Forma: Research Funding; Delta-Fly: Research Funding; Sangamo: Research Funding; Elevate: Research Funding; Biomed Valley Discoveries: Research Funding; Bio: Research Funding; Actuate: Research Funding; Astellas: Honoraria, Research Funding, Speakers Bureau; Abbvie: Research Funding; Leukemia & Lymphoma Society: Research Funding; Ono-UK: Consultancy, Research Funding; Johnson & Johnson: Current equity holder in publicly-traded company; Arog: Research Funding; Trovagene: Research Funding; Tolero: Research Funding; Takeda: Research Funding; Sanofi: Honoraria, Research Funding, Speakers Bureau; Novartis: Consultancy, Research Funding; Pharma: Consultancy; Eli Lilly: Research Funding; ASH foundation: Other: Chair-unpaid; Sellas: Research Funding; Ono: Consultancy; Incyte: Consultancy; Ariad: Research Funding; AstraZeneca: Consultancy; Kaiser Permanente: Consultancy; Cyclacel: Research Funding; MedImmune: Research Funding; Ambit: Research Funding; Boehringer-Ingleheim: Research Funding; Cellerant: Research Funding; CTI Biopharma: Research Funding; Janssen: Research Funding; Kura Oncology: Research Funding; Pharmacyclics: Honoraria, Speakers Bureau; Millennium: Research Funding; National Marrow Donor Program: Research Funding; NIH: Research Funding; Onyx: Research Funding; Pharmamar: Research Funding; UC Davis: Research Funding; UCSD: Research Funding; Evidera: Consultancy; NCI: Consultancy; Novartis: Speakers Bureau. Taparia: Novartis: Consultancy. Verstovsek: Protagonist Therapeutics: Research Funding; Roche: Research Funding; AstraZeneca: Research Funding; Celgene: Consultancy, Research Funding; NS Pharma: Research Funding; PharmaEssentia: Research Funding; CTI BioPharma: Research Funding; Gilead: Research Funding; Incyte Corporation: Consultancy, Research Funding; Ital Pharma: Research Funding; Blueprint Medicines Corp: Research Funding; Promedior: Research Funding; Genentech: Research Funding; Novartis: Consultancy, Research Funding; Sierra Oncology: Consultancy, Research Funding; Constellation: Consultancy; Pragmatist: Consultancy. Khoury: Stemline Therapeutics: Research Funding; Angle: Research Funding; Kiromic: Research Funding. Brooks: Stemline Therapeutics: Current Employment. Mughal: Stemline: Current Employment, Current holder of stock options in a privately-held company; Oxford University Press, Informa: Other: financial benefit and/or patents . Pemmaraju: LFB Biotechnologies: Consultancy; ASCO Leukemia Advisory Panel: Membership on an entity's Board of Directors or advisory committees; Samus: Other, Research Funding; Springer Science + Business Media: Other; MustangBio: Consultancy, Other; Incyte: Consultancy; Novartis Pharmaceuticals: Consultancy, Other: Research Support, Research Funding; HemOnc Times/Oncology Times: Membership on an entity's Board of Directors or advisory committees; ASH Communications Committee: Membership on an entity's Board of Directors or advisory committees; Celgene Corporation: Consultancy; Stemline Therapeutics, Inc.: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; DAVA Oncology: Consultancy; Aptitude Health: Consultancy; Daiichi Sankyo, Inc.: Other, Research Funding; Affymetrix: Consultancy, Research Funding; Abbvie Pharmaceuticals: Consultancy, Membership on an entity's Board of Directors or advisory committees, Other, Research Funding; Protagonist Therapeutics, Inc.: Consultancy; Roche Diagnostics: Consultancy; CareDx, Inc.: Consultancy; Dan's House of Hope: Membership on an entity's Board of Directors or advisory committees; Plexxicon: Other, Research Funding; Sager Strong Foundation: Other; Clearview Healthcare Partners: Consultancy; Blueprint Medicines: Consultancy; Cellectis S.A. ADR: Other, Research Funding; Bristol-Myers Squibb Co.: Consultancy; ImmunoGen, Inc: Consultancy; Pacylex Pharmaceuticals: Consultancy.
Author notes
 This icon denotes a clinically relevant abstract
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal